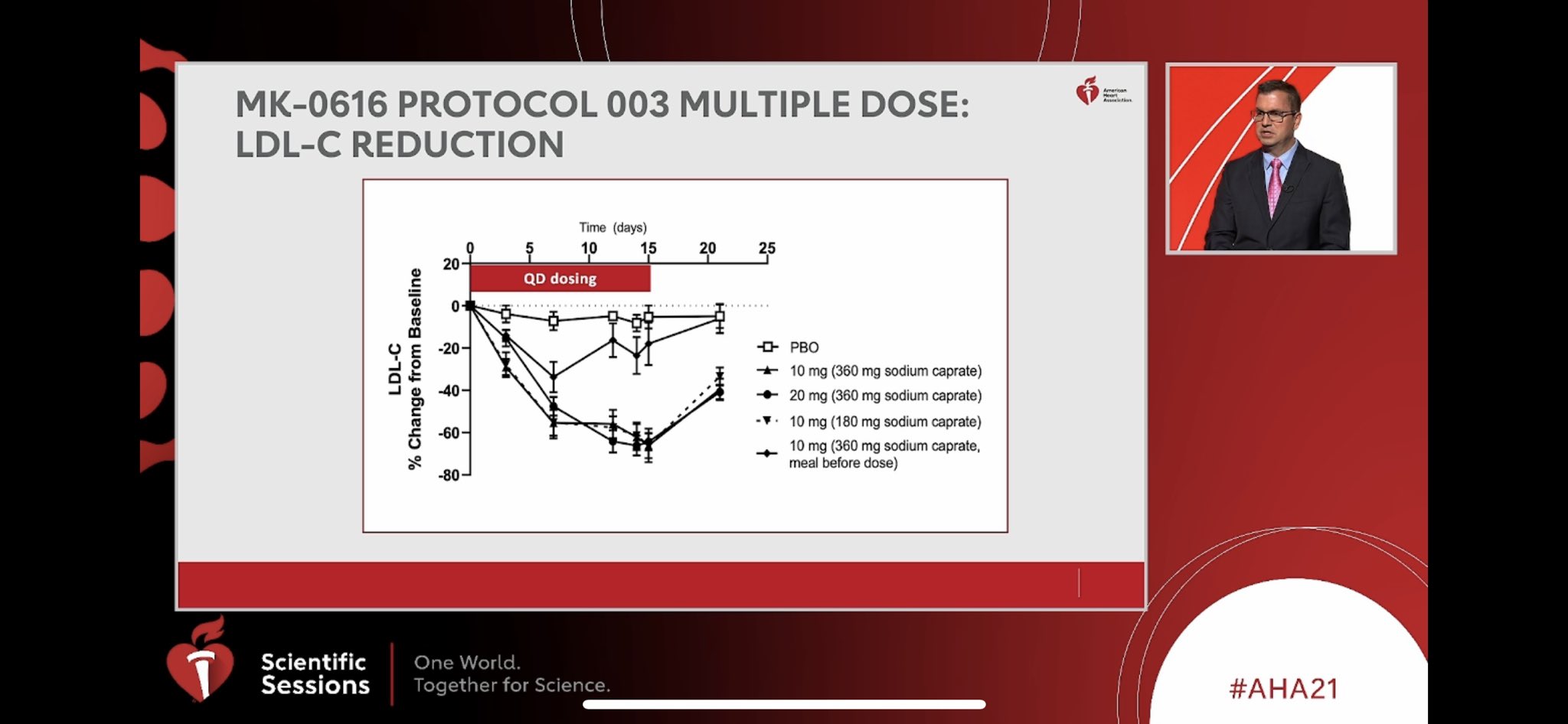
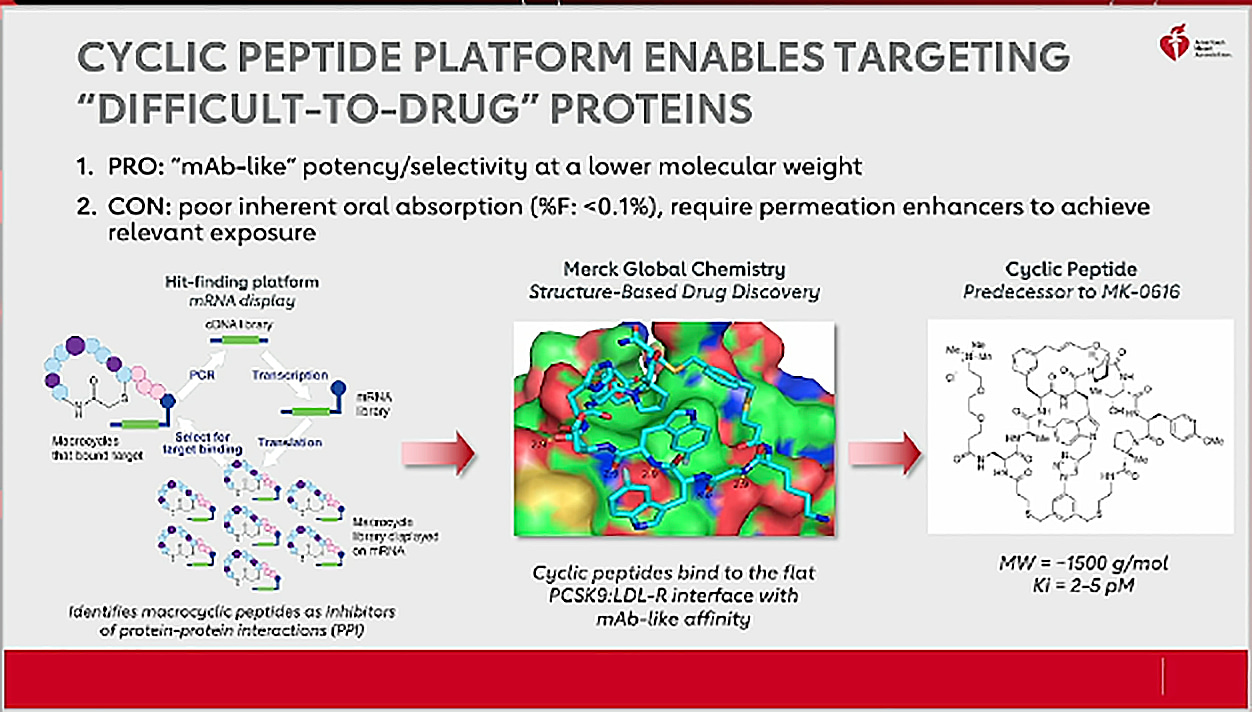
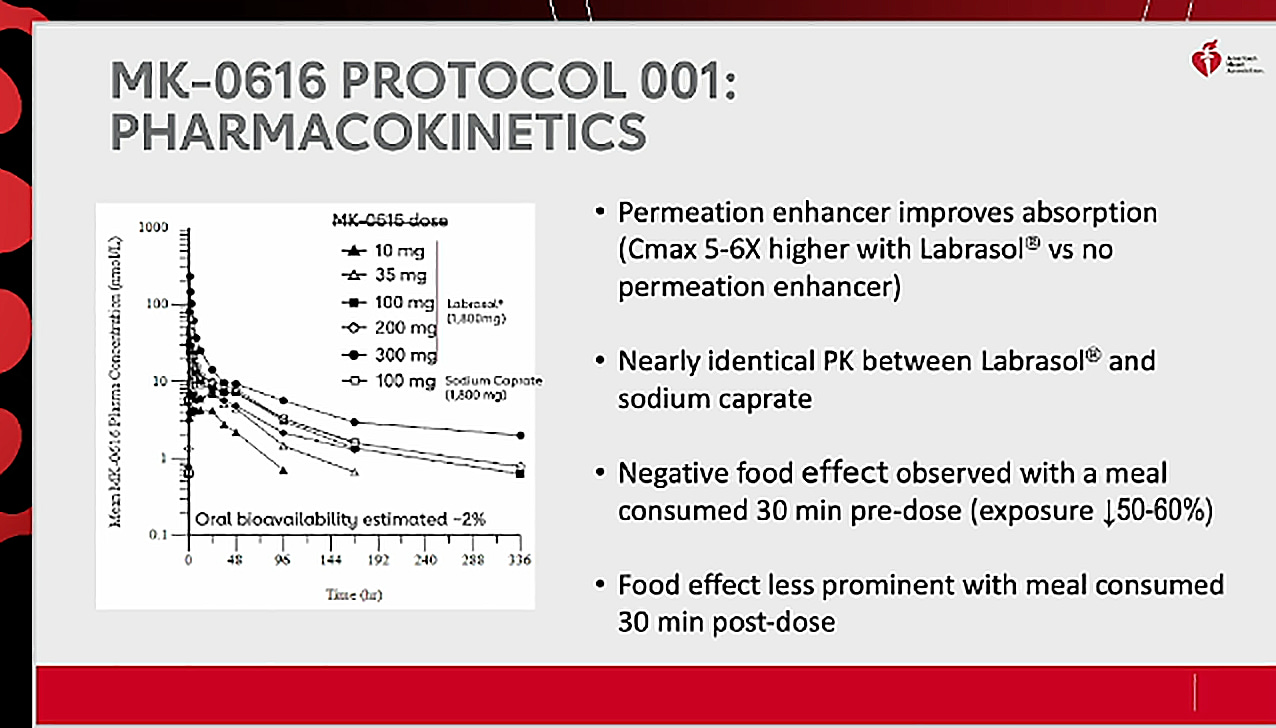
Vanitha M K - Sr.Specialist -Key account management- Customer Excellence - Merck Life Science | LinkedIn

Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer | Journal of Medicinal Chemistry

Chemical structures of M1-mAChR allosteric ligands developed by Merck... | Download Scientific Diagram
Merck & Co., Ridgeback Biotherapeutics, and Drug Innovations at Emory (DRIVE) – Molnupiravir (MK-4482, EIDD-2801)

Chemical structure of compound MK-8931. MK-8931 is developed at Merck... | Download Scientific Diagram

ESMO 2022: Randomized, phase III study of MK-7684A plus concurrent chemoradiotherapy (cCRT) followed by MK-7684A vs cCRT followed by durvalumab for unresectable, locally advanced, stage III non-small cell lung cancer (NSCLC): KEYVIBE-006

Merck Receives Fast Track Designation from the U.S. FDA for MK-2060, an Investigational Anticoagulant Therapy | Business Wire
In Vitro Resistance Analysis of Merck's HCV NS5a Inhibitor MK-8742 Demonstrates Increased Potency AgainstClinical Resistance Variants and Improved Resistance Profile